If you find these posts interesting and think others might do so, spread the word.
Back in the 2009 H1N1 influenza pandemic there was a vaccine safety issue that arose too. In 2010, Finland and Sweden noticed increased cases of narcolepsy, and suspicion fell on Pandemrix, a H1N1 vaccine. A recent systematic review has found that:
During the first year after vaccination, the relative risk of narcolepsy was increased 5 to 14-fold in children and adolescents and 2 to 7-fold in adults. The vaccine attributable risk in children and adolescents was around 1 per 18,400 vaccine doses.
And in the UK, where Pandemrix was also used, found that it occurred in 1 in 34,500 doses according to a 2020 study. It’s still a bit of a mystery about the mechanism for this adverse effect, which has arguably compelling evidence that it is linked with this particular H1N1 vaccine. The adjuvant AS03 is one culprit, although H1N1 itself appears linked to narcolepsy as well. So we can’t discount adjuvants and other excipient issues from the causal mechanism of vaccine adverse effects…
Science has an interesting piece on the potential for preservatives and proteins to have potentially caused the unusual clotting in some individuals.
Both J&J and AstraZeneca use modified adenoviruses to deliver and express the spike protein gene of SARS-CoV-2. But new data posted Tuesday in a preprint on Research Square show that doses of the AstraZeneca vaccine also contain significant amounts of protein from human cells—presumably from the human cell line used to grow the virus during the manufacturing process. The preprint’s authors, some whom were among the first to identify the VITT side effect, propose that these proteins, together with another component of the vaccine called ethylenediaminetetraacetic acid (EDTA), may set off a dangerous response by the immune system in some vaccine recipients.
Again, a lot of this is unproven as of yet, and it is still worth remembering that these unusual clotting events remain extremely rare events. The MHRA’s latest data for the AZ vaccine is 168 cases with 32 deaths, 1 from 2nd dose (no change, and this case had other reasons for the clotting event). 7.9 reported cases per million. This does not change previous MHRA advice, and it remains and extremely rare event (roughly 1 in 125,000). Their overall view remains the same: 'On the basis of this ongoing review, the advice remains that the benefits of the vaccine outweigh the risks in the majority of people'.
The US has also unpaused the J&J vaccine, with 15 reported cases out of 8 million doses given. So they have unpaused the vaccine despite more cases being found. Which really makes you wonder if they should had paused in the first place after the initial few cases. Such a pause will have damaged vaccine confidence, and protestations that the pause will have increased confidence in the vaccine’s safety seem misplaced to me. There is no evidence that that is the right approach, and contrary examples in Europe over the AZ vaccine. In the UK, where a pause was not used, confidence in the AZ vaccine is holding up.
The messaging about AZ vaccine from European state regulators is already having a direct effect on the most vunerable. Haiti has declined to accept over 750,000 doses of AZ vaccine. Not because they have concerns about safety, but because the population’s view of the vaccine now is such that they would prefer to use a different vaccine.
Ten fold errors in Children by Christine Parry.
Fatal adverse drug reactions: A worldwide perspective in the World Health Organization pharmacovigilance database by Montastruc et al.
The Antiscience Movement Is Escalating, Going Global and Killing Thousands by Peter J Hotez
NOTE: This last piece mainly look at the rise of 'anti-science' in some of the US right. However, the issue isn't anti-science itself, but the identity driven tribalism that puts politics into some areas of science. Similar rejections, about different science, also occurs on some of left too.
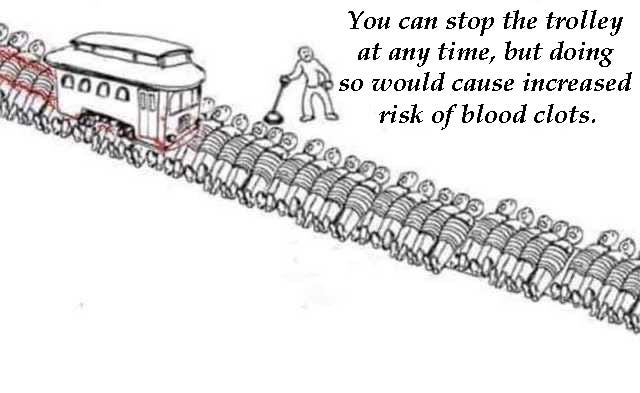
And this Trolley Problem meme is excellent on risk….
While we are on this matter again, I think the public acceptance of risk hasn't changed that much. In the UK, AZ vaccine is still being widely accepted in a highly successful vaccination programme. So, if communicated well, the public can still manage extremely rare risks, in a vaccine that carries huge benefits. However, that may have varied in different countries. In some countries with a legacy that may have created already lowered trust in vaccines, and that have had pauses and political statements about a vaccine, things may be different. The AZ vaccine is starting to look like a vaccine that the “West” is not using, but sending off elsewhere. This is regrettable. There’s a lot of talk about vaccine equity in relation to supply, but I think vaccine equity in terms of risk is something we might consider.
I have seem individual EU citizens saying they would happily have the AZ vaccine, despite not being eligible due to the age-related restrictions brought in. So perhaps the risk aversion isn’t from the public, but EU state regulators who have put restrictions in place - some for valid epidemiological reasons (even if EMA has not). After a year of missteps, perhaps an ultra-cautious approach to risk is appropriate - although the UK had its own share of missteps in the opening of this pandemic, so that acts as a counter-example. The MHRA appears to have stuck to its scientific processes, as has the EMA.
At some point this newsletter will stop focusing on vaccines… The next month is going to be pretty busy for for me at work, so the letter may be brief.
That’s all and stay safe.
Anthony
Don’t forget to report your suspected adverse effects from medicines and vaccines. In the UK, this means using the Yellow Card Scheme.