Is it Safe? Vaccination safety plans
Yep, everyone has a plan until they are punched in the face.
If you find this post interesting and think others might do so, spread the word. [I added the news about France and Germany on the 15th March 2021, post publication]
I thought it was interesting to get some quick thoughts down about the recent suspension in use of the Astra-Zeneca (AZ) vaccine in some EU states, despite the European Medicines Agency’s confidence in the vaccine. So this post is about some of the decision making being made in the light of the concerns about reports of thrombosis after vaccination, how regulatory convergence seems further away than ever, and how misapplication of the precautionary principle is not the cost-free 'erring on the side of caution' option that some seem to think it is.
Let's start by saying that vaccines are a nightmare for regulatory agencies. They provide a future benefit, and immediate risks (even if small). If you have heart failure or cancer, you might be concerned about the risk of side effects from a treatment, but you know that you want the benefit. For a vaccine, the benefit follows an infection the person may or may not get - we'll leave aside the wider benefits of reduced transmission and herd immunity for simplicity.
In the case of COVID-19 vaccines, the high prevalence of cases and a relatively high mortality rate make the decision clear enough for most people, especially with the high effectiveness of all the vaccines we are seeing. Again, we'll leave to one side other motivations that the vaccination campaign also promises. The 'return to normal'. Meeting your family. Not teaching students via Zoom (a big personal driver of mine there).
In the face of a global pandemic, the largely mild transient side effects of the vaccines are a price that most people think are worth paying (to varying degrees, but even the low uptake groups in the UK are over 50%).
However, a long history of vaccines scares and concerns have existed since the invention of vaccination. All drugs have an effect on your body, but there is something about vaccines that seems more invasive. This means that decision making and communication about the safety of vaccines is fraught. Every step needs to be taken carefully, and disaster can strike when poor decisions are made. Think Indiana Jones working his way across a floor that triggers poison arrows, only to blow it when switching his bag of sand for a gold statue.
Regulatory agencies have been thinking about how to create public confidence in vaccines for months. Both the UK's MHRA and the EU's EMA have extensive plans for pharmacovigilance for these vaccines. This is to ensure that we can monitor the ongoing safety of the vaccines in the population, but also to ensure that public confidence in the systems for monitoring the vaccines is high. Both have tried to involve the public and to be transparent about reported harms of vaccines. Both have set up new systems and studies to look for safety signals.
For years there has also been interest in global convergence in pharmacovigilance and drug safety, to try and align regulators like EMA and the FDA. You can also see this in the arguments from some that the FDA ought to licence the AZ vaccine in the states. As recently at the end of February 2021 the International Coalition of Medicines Regulatory Authorities (ICMRA) met to discuss the global response to COVID-19 and International collaboration on vaccine safety.
A workshop was held:
'The participants of this workshop stressed that joint work among regulators was needed in order to learn from each other, take appropriate mitigating measures (where needed) and communicate in a consistent way across regions to build and maintain public trust in vaccines.'
There was support for a Tabletop Simulation exercise to look for real areas for co-operation:
'This could look on a known suspected adverse event of special interest as a test case, for example Bell’s palsy, or the alert raised when Norway reported deaths in frail elderly vaccinees. Other comments related to the importance of collaboration to understand better background incident rates, as well as differential benefit-risk for different populations and the importance of consistent responses at global level.'
The Norwegian frail patient story noted above was an early COVID-19 vaccine story, which while important, did not seem to expose major differences in approach.
So the good news first, in response to the Danish decision to withdrawn the AZ vaccine the EMA's Pharmacovigilance Risk Assessment Committee (PRAC) are currently investigating the cases of thromboembolic events, and in a recent press release (11/3/2021) emphasised the benefits of the AZ vaccine outweighed the risks.
'There is currently no indication that vaccination has caused these conditions, which are not listed as side effects with this vaccine. The position of EMA’s safety committee PRAC is that the vaccine’s benefits continue to outweigh its risks and the vaccine can continue to be administered while investigation of cases of thromboembolic events is ongoing.'
This aligns with the UK's MHRA view published on the same day.
'This is a precautionary measure by the Danish, Norwegian and Icelandic authorities. It has not been confirmed that the report of a blood clot was caused by the AstraZeneca COVID-19 Vaccine. People should still go and get their COVID-19 vaccine when asked to do so.'
The key point is that 'Reports of blood clots received so far are not greater than the number that would have occurred naturally in the vaccinated population.' In the UK over 11 million doses of Az have been used, but there is no signal in the UK data.
The WHO have said that the Astra-Zeneca COVID-19 vaccine should continue to be used since there is no indication that this risk is true.
So, we have convergence and a good message on this issue.
But something has gone wrong in the EU.
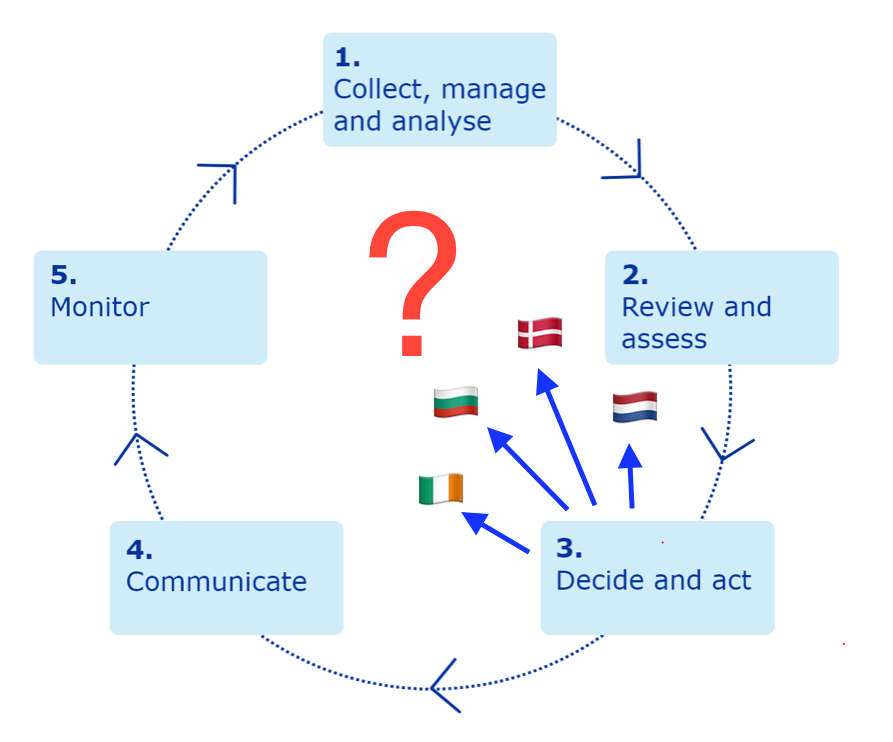
The Netherlands, Bulgaria, Denmark, and Ireland have all paused the use of the AZ vaccine. So have Iceland and Norway. While the latter aren't EU states, they are somewhat tied into EU, since marketing authorisations from the EU are valid in all EU states and European Economic Area (EEA) and European Free Trade Association (EFTA) states, and also their decisions are reported in the media as "European". Austria stopped the use of a single batch after one recipient had a clot, and Estonia, Latvia, Lithuania and Luxembourg stopped using that same batch. Others, such as Germany, are sticking with the AZ vaccine. The German Health minister Jen Spahn saying 'From what we know so far, the benefit... is far greater than the risk'. [UPDATE 15/3/2021 Germany has now suspended the AZ vaccine, and Macron has annouced the suspension in France]

The issue seems to be a misapplication of the precautionary principle. Here's Ireland's Deputy Chief Medical Officer:
'Dr Glynn added that he hoped there will be more "reassuring data" this week and that the programme can restart.
"It may be nothing, we may be overreacting and I sincerely hope that in a week's time we are accused of being overcautious," he said.'
The Dutch also cite the precautionary principle.

The precautionary principle, which originally came from environmental concerns, is not an easy fit to pharmacovigilance. An excellent article by Callréus covers this, noting that its use would lead to false positive signals.
Given the large number of false positive signals generated by various data-mining strategies, a parsimonious application of the precautionary principle would be warranted in this area or else the number of market withdrawals would be unreasonable.
So we need to apply the precautionary principle to the precautionary principle when it comes to drug safety. Callréus argued the precautionary principle needs to be adapted for drug safety use:
'Originating from a criticism of traditional risk assessment, the key element of the precautionary principle is the justification for acting in the face of uncertain knowledge about risks. More recent is its appearance in public health and in relation to drug safety issues. Rather than either embracing or rejecting the precautionary principle, studying the experience gained from its previous applications should be the way forward. If believed to be of relevance, in order to avoid arbitrary and unpredictable decision making, its interpretation and possible application need to be adapted to the conditions of pharmaceutical risk management.'
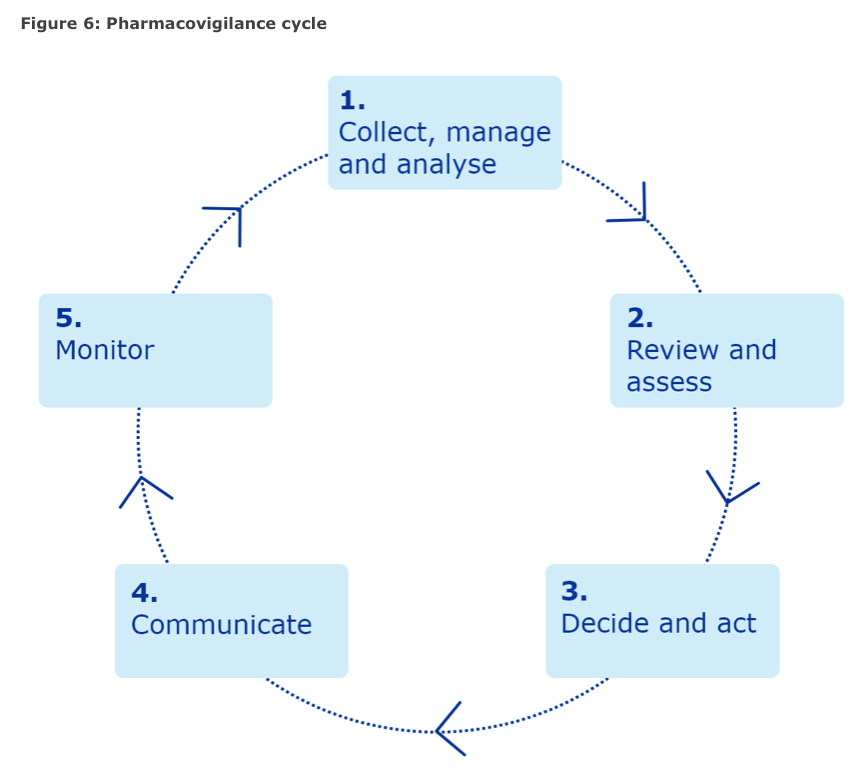
The EMA COVID-19 vaccine plan included their safety monitoring and risk management plan and they helpfully include a pictorial pharmacovigilance cycle.
Here it is.
Sadly, it now seems to be working like this.
It feels like trust in the institutions of EU, have been damaged following the failure of the vaccination programme.
What is the point of having a European Medicines Agency with well thought through plans on vaccine safety if individual EU states are going to do knee-jerk decisions, misapplying the precautionary principle?
In two weeks time, how much confidence will the public have in the AZ vaccine it starts to be used again by states that suspended it on poor evidence? Confidence in the AZ vaccine is already low in the EU with 1 in 4 refusing it, in preference for the Pfizer-BioNTech vaccine. ‘Erring on the side of caution’ is reducing public confidence in vaccines in a pandemic?
What will the effect of this be in the UK, Germany and other states that do back the AZ vaccine? A hyper-connected world, where information is spread on social networks cross borders means that these decisions are not contained within the countries where they are taken. More worryingly, what effect will this have on vaccine hesitancy in countries receiving the AZ vaccine via COVAX? What effect will it have on the public view of vaccination safety in general?
How can you have global regulatory and pharmacovigilance convergence when the decisions of the EMA are second guessed by individual governments? Does local political accountability means that such convergence can only ever be limited in nature?
All the plans in the Pharmacovigilance Plan of the EU Regulatory Network for COVID-19 Vaccines seem to have been ignored as individual members of the EU, arguably with less expertise than EMA go their own way.
Everyone's got a plan until they are punched in the face as they say, and this pandemic has dealt a right hook to rational decision making about vaccine safety.
That’s all for this week and stay safe.
Anthony
Don’t forget to report your suspected adverse effects from medicines and vaccines. In the UK, this means using the Yellow Card Scheme.